5 Covid Testing Paperwork Facts

Introduction to Covid Testing Paperwork
The COVID-19 pandemic has brought about a plethora of changes in how we live, work, and interact with each other. One of the critical aspects of managing the pandemic is testing, which helps in identifying infected individuals and thereby preventing the spread of the virus. However, with testing comes a significant amount of paperwork, which can be overwhelming for both healthcare providers and patients. In this article, we will delve into five essential facts about COVID-19 testing paperwork, aiming to clarify the process and highlight its importance.
Fact 1: Informed Consent

When it comes to COVID-19 testing, informed consent is a crucial piece of paperwork. This document ensures that the patient is fully aware of the test’s purpose, the procedures involved, potential risks, and the implications of the test results. It’s a legal requirement that protects both the patient and the healthcare provider. The informed consent form typically includes details about the type of test being administered (e.g., PCR, antigen, or antibody test), how the sample will be collected (e.g., nasal swab, blood sample), and what the patient can expect during and after the testing process.
Fact 2: Test Result Reporting
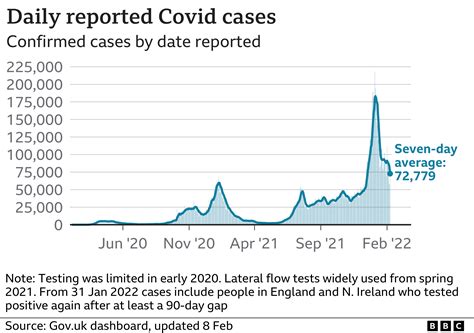
Another critical aspect of COVID-19 testing paperwork is the reporting of test results. Laboratories and healthcare facilities are required to report positive test results to local and national health authorities. This reporting is essential for tracking the spread of the virus, identifying hotspots, and implementing appropriate public health measures. The paperwork involved in reporting includes filling out specific forms with patient details, test results, and other relevant information. This process is highly regulated to ensure patient privacy and confidentiality while also facilitating the collection of vital epidemiological data.
Fact 3: Insurance and Billing
The financial aspect of COVID-19 testing also involves a considerable amount of paperwork. In many countries, COVID-19 tests are covered by insurance, but the process of billing and reimbursement can be complex. Patients may need to fill out forms to submit to their insurance providers, and healthcare providers must navigate the billing process to receive compensation for the tests they administer. Understanding the billing codes and the specific requirements for insurance reimbursement is crucial for avoiding delays or denials of claims.
Fact 4: Data Privacy and Security
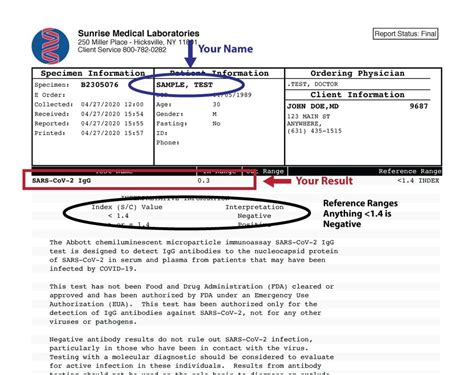
Given the sensitive nature of health information, COVID-19 testing paperwork must adhere to strict guidelines regarding data privacy and security. The Health Insurance Portability and Accountability Act (HIPAA) in the United States, and similar regulations in other countries, dictate how patient information can be collected, stored, and shared. This includes ensuring that all paperwork and digital records are handled in a way that protects patient confidentiality and complies with legal standards.
Fact 5: Variability by Location

Lastly, it’s essential to note that the specific requirements for COVID-19 testing paperwork can vary significantly by location. Different countries, states, or even local jurisdictions may have their own regulations, forms, and procedures for testing and reporting. This variability can make it challenging for healthcare providers and patients to navigate the system, especially in cases where individuals are tested while traveling. Being aware of these local requirements and ensuring compliance is crucial for smooth operation and for contributing to global efforts to combat the pandemic.
📝 Note: Staying informed about local health regulations and guidelines is key to understanding the specific paperwork requirements for COVID-19 testing in your area.
As we reflect on these facts, it becomes clear that COVID-19 testing paperwork plays a vital role in the management of the pandemic. From informed consent to data privacy, each aspect of the paperwork process contributes to a broader strategy of controlling the virus’s spread and ensuring public health. By understanding and complying with these requirements, we can work together more effectively to navigate the challenges posed by COVID-19.
What is the purpose of informed consent in COVID-19 testing?

+
The purpose of informed consent is to ensure that patients are fully aware of the testing procedure, its risks, and the implications of the results, thereby protecting both the patient and the healthcare provider.
Why is reporting of test results important?

+
Reporting of test results is crucial for tracking the spread of the virus, identifying areas with high infection rates, and implementing appropriate public health measures to control the pandemic.
How does insurance cover COVID-19 testing?
+
In many countries, COVID-19 tests are covered by insurance. However, the specifics of coverage, including any out-of-pocket costs for the patient, can vary depending on the insurance provider and the patient’s policy.



