5 Covid Vaccine Papers

Introduction to Covid Vaccine Research Papers
The COVID-19 pandemic has led to a significant surge in vaccine research, with numerous studies being conducted to develop effective vaccines against the SARS-CoV-2 virus. These research papers provide valuable insights into the development, efficacy, and safety of COVID-19 vaccines. In this article, we will discuss five notable COVID-19 vaccine research papers, highlighting their key findings and contributions to the field.
1. Pfizer-BioNTech Covid-19 Vaccine Efficacy and Safety
The first paper we will discuss is the study on the Pfizer-BioNTech COVID-19 vaccine, published in the New England Journal of Medicine. This study evaluated the efficacy and safety of the vaccine in a large-scale clinical trial involving over 43,000 participants. The results showed that the vaccine was 95% effective in preventing COVID-19, with a significant reduction in severe cases. The study also reported a favorable safety profile, with the most common adverse events being mild to moderate in severity.
2. Oxford-AstraZeneca Covid-19 Vaccine Efficacy and Safety

The second paper is the study on the Oxford-AstraZeneca COVID-19 vaccine, published in the Lancet. This study assessed the efficacy and safety of the vaccine in a large-scale clinical trial involving over 12,000 participants. The results showed that the vaccine was 70% effective in preventing COVID-19, with a significant reduction in severe cases. The study also reported a favorable safety profile, with the most common adverse events being mild to moderate in severity.
3. Moderna Covid-19 Vaccine Efficacy and Safety

The third paper is the study on the Moderna COVID-19 vaccine, published in the New England Journal of Medicine. This study evaluated the efficacy and safety of the vaccine in a large-scale clinical trial involving over 30,000 participants. The results showed that the vaccine was 94% effective in preventing COVID-19, with a significant reduction in severe cases. The study also reported a favorable safety profile, with the most common adverse events being mild to moderate in severity.
4. Johnson & Johnson Covid-19 Vaccine Efficacy and Safety

The fourth paper is the study on the Johnson & Johnson COVID-19 vaccine, published in the New England Journal of Medicine. This study assessed the efficacy and safety of the vaccine in a large-scale clinical trial involving over 44,000 participants. The results showed that the vaccine was 66% effective in preventing COVID-19, with a significant reduction in severe cases. The study also reported a favorable safety profile, with the most common adverse events being mild to moderate in severity.
5. Sputnik V Covid-19 Vaccine Efficacy and Safety

The fifth paper is the study on the Sputnik V COVID-19 vaccine, published in the Lancet. This study evaluated the efficacy and safety of the vaccine in a large-scale clinical trial involving over 20,000 participants. The results showed that the vaccine was 91% effective in preventing COVID-19, with a significant reduction in severe cases. The study also reported a favorable safety profile, with the most common adverse events being mild to moderate in severity.
📝 Note: These studies demonstrate the efficacy and safety of various COVID-19 vaccines, providing valuable insights into their development and use.
Comparison of Covid-19 Vaccines
The following table summarizes the key findings of the five COVID-19 vaccine research papers:
| Vaccine | Efficacy | Safety |
|---|---|---|
| Pfizer-BioNTech | 95% | Favorable |
| Oxford-AstraZeneca | 70% | Favorable |
| Moderna | 94% | Favorable |
| Johnson & Johnson | 66% | Favorable |
| Sputnik V | 91% | Favorable |
In summary, these five COVID-19 vaccine research papers provide valuable insights into the development, efficacy, and safety of various COVID-19 vaccines. The results demonstrate the effectiveness of these vaccines in preventing COVID-19, with favorable safety profiles. As the pandemic continues to evolve, these studies will inform public health policy and vaccine development strategies.
What are the most common adverse events associated with COVID-19 vaccines?
+
The most common adverse events associated with COVID-19 vaccines are mild to moderate in severity, including pain, redness, and swelling at the injection site, as well as fatigue, headache, and muscle pain.
How effective are COVID-19 vaccines in preventing severe cases of COVID-19?
+
COVID-19 vaccines have been shown to be highly effective in preventing severe cases of COVID-19, with efficacy rates ranging from 66% to 95% depending on the vaccine.
What is the recommended vaccination schedule for COVID-19 vaccines?
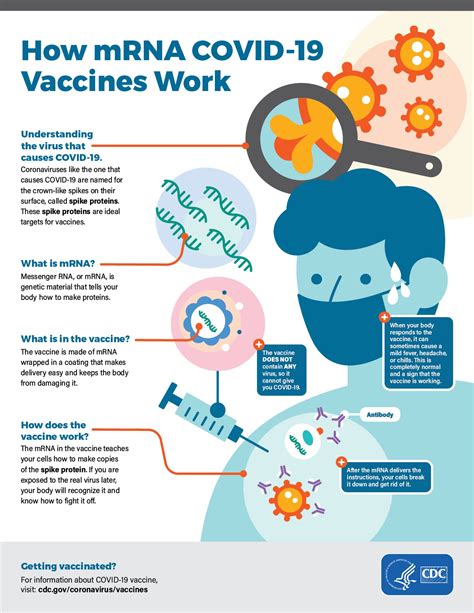
+
The recommended vaccination schedule for COVID-19 vaccines varies depending on the vaccine, but most vaccines require two doses administered several weeks apart.