5 Vaccine Paperworks Needed
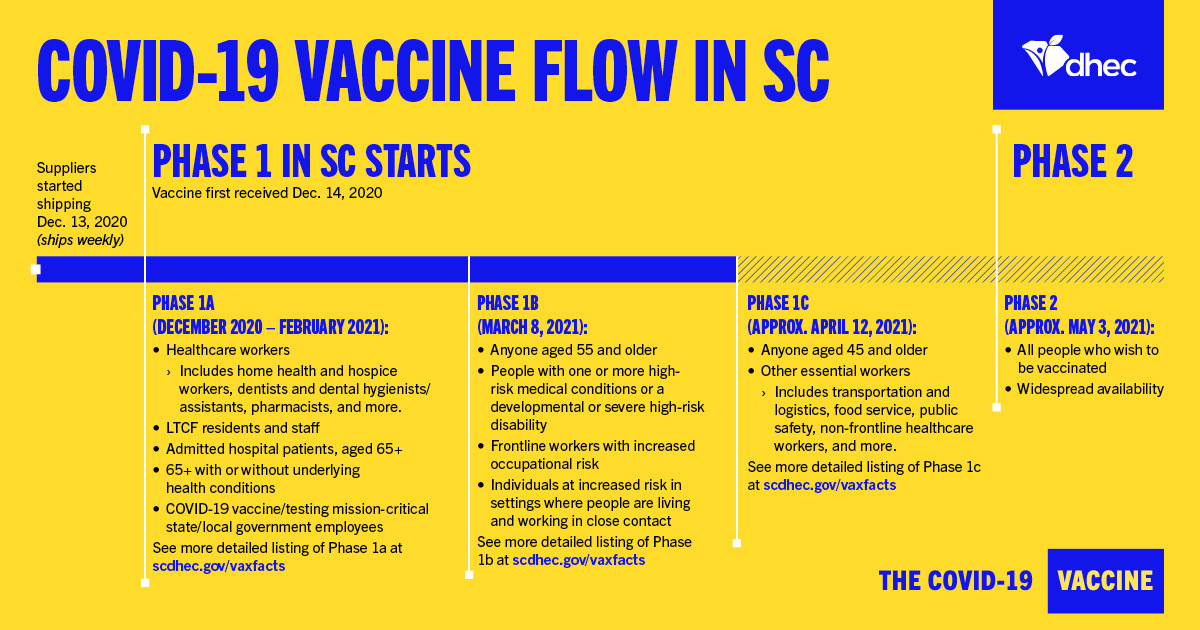
Introduction to Vaccine Paperworks

When it comes to ensuring public health and safety, vaccines play a crucial role. However, the process of developing, distributing, and administering vaccines involves a significant amount of paperwork. This paperwork is essential for tracking vaccine safety, efficacy, and distribution. In this article, we will explore the five key vaccine paperwork needed for a successful vaccine program.
Understanding the Importance of Vaccine Paperwork

Vaccine paperwork is not just a bureaucratic requirement; it serves several critical purposes. It helps in tracking vaccine batches, monitoring vaccine safety, and ensuring compliance with regulatory requirements. Moreover, it facilitates the investigation of adverse events and enables public health officials to make informed decisions. With the rise of digital health records, the management of vaccine paperwork has become more efficient, but the fundamental need for accurate and comprehensive documentation remains unchanged.
1. Vaccine Administration Record (VAR)
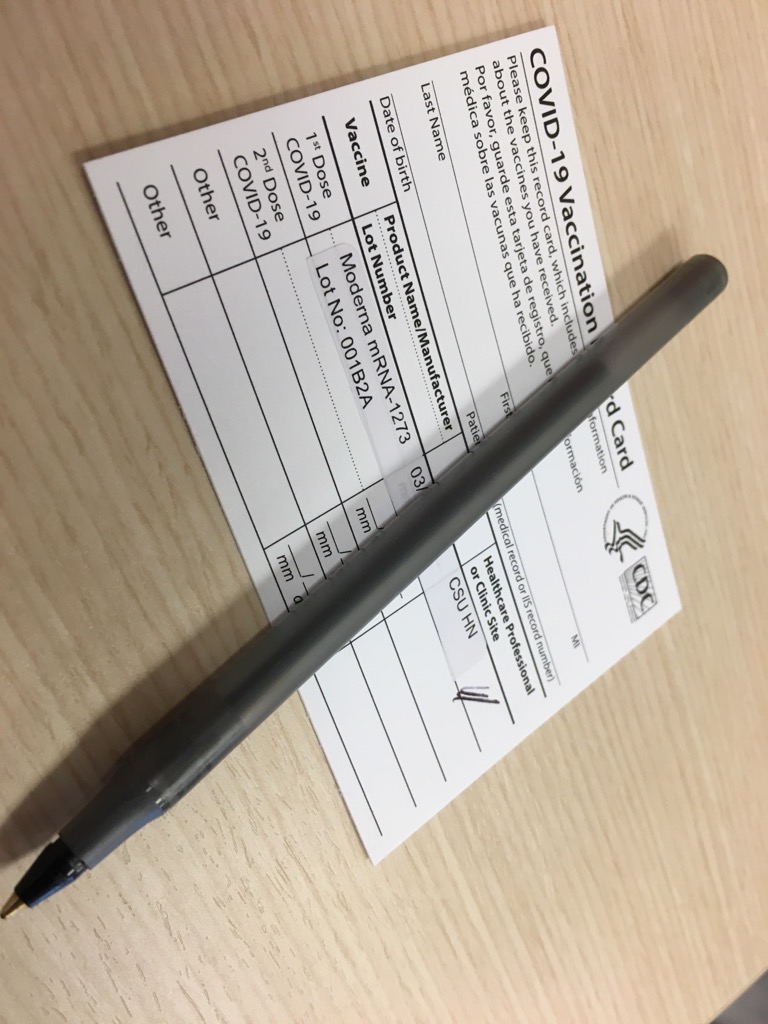
The Vaccine Administration Record (VAR) is a crucial document that records the details of vaccine administration, including the vaccine type, date of administration, dose number, and route of administration. This record is essential for tracking individual vaccination histories and ensuring that patients receive the recommended vaccine doses. Healthcare providers must maintain accurate and up-to-date VARs to facilitate continuity of care and prevent vaccine-related errors.
2. Vaccine Storage and Handling Record
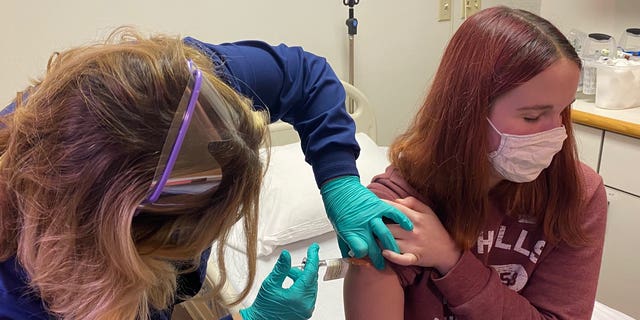
The Vaccine Storage and Handling Record is vital for ensuring that vaccines are stored and handled properly. This record documents the storage conditions, temperature monitoring, and handling procedures for vaccines. It helps in preventing vaccine spoilage and ensures that vaccines remain potent and effective. By maintaining accurate storage and handling records, healthcare providers can minimize the risk of vaccine-related errors and ensure the integrity of the vaccine supply chain.
3. Vaccine Inventory Management Record
The Vaccine Inventory Management Record is used to track vaccine inventory levels, including the quantity of vaccines in stock, vaccine expiration dates, and vaccine distribution records. This record helps healthcare providers to manage vaccine supplies efficiently, prevent stockouts, and ensure that vaccines are available when needed. By maintaining accurate inventory records, healthcare providers can also identify trends in vaccine usage and plan for future vaccine needs.
4. Adverse Event Reporting (AER) Form
The Adverse Event Reporting (AER) form is used to document and report adverse events related to vaccine administration. This form includes information about the adverse event, vaccine administered, and patient demographics. The AER form is essential for monitoring vaccine safety and enabling public health officials to investigate adverse events and take corrective actions. By reporting adverse events, healthcare providers can contribute to the ongoing evaluation of vaccine safety and efficacy.
5. Vaccine Information Statement (VIS)
The Vaccine Information Statement (VIS) is a document that provides patients with information about the benefits and risks of vaccines, vaccine ingredients, and potential side effects. The VIS is essential for informed consent and enables patients to make informed decisions about their vaccination. Healthcare providers must provide patients with the VIS before administering a vaccine, and patients must sign the document to acknowledge that they have received the information.
📝 Note: Healthcare providers must ensure that all vaccine paperwork is accurate, complete, and up-to-date to ensure the success of vaccine programs and maintain public trust in vaccine safety and efficacy.
In summary, the five key vaccine paperwork needed for a successful vaccine program are the Vaccine Administration Record, Vaccine Storage and Handling Record, Vaccine Inventory Management Record, Adverse Event Reporting form, and Vaccine Information Statement. These documents are essential for ensuring vaccine safety, efficacy, and distribution, and healthcare providers must maintain accurate and comprehensive records to facilitate continuity of care and prevent vaccine-related errors. By understanding the importance of vaccine paperwork, healthcare providers can contribute to the ongoing evaluation of vaccine safety and efficacy, and ultimately, protect public health and safety.
What is the purpose of the Vaccine Administration Record (VAR)?

+
The Vaccine Administration Record (VAR) is used to record the details of vaccine administration, including the vaccine type, date of administration, dose number, and route of administration. It helps in tracking individual vaccination histories and ensuring that patients receive the recommended vaccine doses.
Why is the Vaccine Storage and Handling Record important?
+
The Vaccine Storage and Handling Record is important because it helps in preventing vaccine spoilage and ensures that vaccines remain potent and effective. By maintaining accurate storage and handling records, healthcare providers can minimize the risk of vaccine-related errors and ensure the integrity of the vaccine supply chain.
What is the purpose of the Adverse Event Reporting (AER) form?

+
The Adverse Event Reporting (AER) form is used to document and report adverse events related to vaccine administration. It helps in monitoring vaccine safety and enabling public health officials to investigate adverse events and take corrective actions.



